Keywords
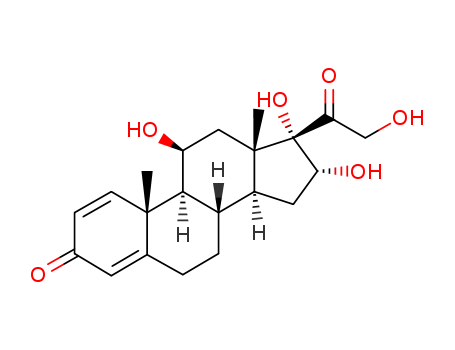
- 11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione
- 16α-Hydroxyprednisolone
- Lower Price 11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione
Quick Details
- ProName: CAS 13951-70-7 16α-Hydroxyprednisolone...
- CasNo: 13951-70-7
- Molecular Formula: C21H28O6
- Appearance: White to off-white powder
- Application: Glucocorticoid API intermediates are t...
- DeliveryTime: 3-7days
- PackAge: bag or barrel or drum
- Port: Shanghai or Shenzhen or Qingdao
- Purity: 99%+
- Storage: Store at room temperature
- Transportation: by express or air or sea
- LimitNum: 0
- Related Substances: <0.001%
- Residue on Ignition: <0.001%
Superiority
1, We offer competitive factory price for both wholesalers and retailers.
2, Fast reply for inquiries.
3, Stable Quality, high purity.
4, We offer EXW/FOB/CFR/DAP/DDP trade terms for your option.
5, Fast Delivery for shipment and Safe for destination custom clearance.
6, Free samples.
7, Free custom clearance with freight prepaid, delivery door to door.
8, More than 22 years export experience in this field.
Details
16a-Hydroxyprednisolone, an intermediate of glucocorticoid APIs, is an intermediate for the synthesis of approved neded drugs including triamcinolone acetonide, budesonide, ciclesonide and desonide. Nide drugs are inhaled glucocorticoids, which have the characteristics of topical medication, low dose, strong local anti-inflammatory effect, and small systemic side effects, and are widely used in the treatment of intractable asthma and inflammation. Prednisolone is a synthetic intermediate-acting glucocorticoid, similar to prednisone, with anti-inflammatory and anti-allergic effects. It has strong anti-inflammatory effects and weak water and salt metabolism. This product is a hydroxy compound (the hydroxy group is at the 11th position), which has a good local application effect and is also suitable for patients with hepatic insufficiency. Oral absorption is fast and complete, reaching the peak plasma concentration within 1 hour, the plasma protein binding rate is 90% to 95%, and the half-life is about 200 minutes. It can enter the fetal circulation, and the amount of the drug that appears in the milk is 0.07% to 0.23%. It is metabolized in the liver and excreted mainly from the kidney, of which the prototype drug accounts for more than 20%. It is suitable for the treatment of allergic diseases such as eczema and dermatitis, and connective tissue diseases such as lupus erythematosus and dermatomyositis.